|
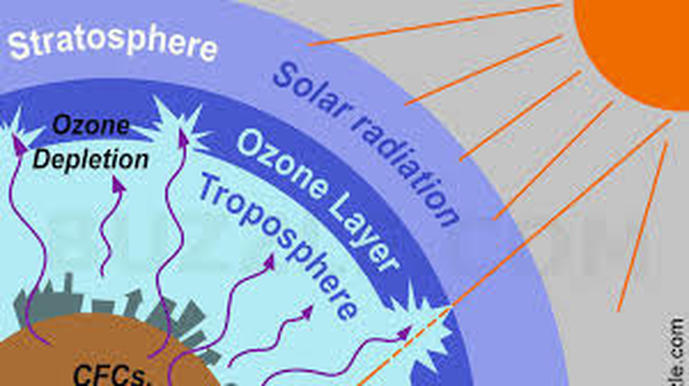
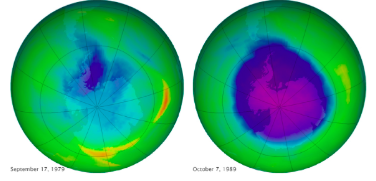
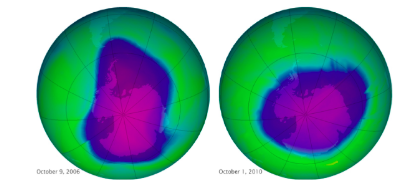
By: I. Weber What is it? Ozone depletion is the phenomenon by which 4% of the ozone layer is reduced per decade. There is a huge amount of ozone depletion on the Earth’s Polar regions, as the lack of sunlight causes ozone to build up in the stratosphere, which is very easily broken down: The “Ozone hole”: a region in the Earth’s stratosphere where there is severe ozone depletion. At the time this image was taken by NASA, the Antarctic ozone hole was almost 30km2. The gradual increase in the Ozone hole serves as evidence to the reduction of the stratospheric ozone layer, shown by the images below: Why is it a problem?
Even though Ozone is a toxic gas, it is our natural sunscreen, as it absorbs ultra-violet radiation from the sun in the stratosphere during the continuous cycle of making and breaking down ozone molecules. This way, the ultra-violet (UV-B) radiation is prevented from reaching the troposphere, where exposure to humans would cause harmful effects, including sunburns, premature aging of the skin, eye damage, and even skin cancer. UV-B radiation also affects the physiological and growth processes in plants and some marine animals, as well as the orientation and mobility of phytoplankton (the base of most aquatic food webs), resulting in elevated death rates and disrupted ecosystems. This way, the depletion of the ozone layer leads to a lack of evolution and development, as well as a significantly reduced range of organisms on Earth. Increased UV-B radiation reaching the Earth’s surface makes plants and agricultural crops less productive, leading to a huge reduction in crop yields, which causes significant economic losses for agricultural industries. As the ozone layer is broken down, the amount of ozone present in the troposphere increases. There, it is regarded as a greenhouse gas, that adds to global warming and climate change. What causes it? Throughout the years, scientists have narrowed down the leading cause of ozone depletion to one human activity: many industries extensively use CFCs (chlorofluorocarbons) and NOx (oxides of nitrogen) in the manufacture of their products. They are pushed by winds into the stratosphere over time. In the stratosphere, solar ultra-violet radiation causes CFCs and NOx to break down ozone as well as prevent its future production. The production and emission of CFCs and NOx is the leading cause of ozone layer depletion (account for almost 80% of the total reduction of ozone). Images from NASA
0 Comments
Leave a Reply. |
Categories
All
Archives
June 2024
|